Retropubic Mid-Urethral Synthetic Sling Procedure
Placement of a minimally invasive polypropylene sling in the retropubic space with a flexible cystoscopy.
Also view the section on urinary incontinence in the TAB above.
Why is it done?
- Stress urinary incontinence.
- A combination of stress incontinence and lesser degree of detrusor overactivity – mixed incontinence.
- Involuntary urine leakage with any exertion, coughing or sneezing.
- Risk factors:
- More than 2 pregnancies, big babies.
- Complicated deliveries, episiotomy.
- Smokers.
- Being overweight.
- Diabetes
- Where Intrinsic Sphincter Deficiency has been proved due to a failed previous sling.
How is it done?
 This procedure is done under a spinal / general anesthetic, as decided by the anesthetist.
This procedure is done under a spinal / general anesthetic, as decided by the anesthetist.
- The legs will be elevated into the lithotomy position.
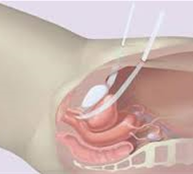
- A small incision is made in the vagina. The sling is placed behind the pubic bone and brought to the skin above the pubic bone, with a small incision.
- A synthetic mesh is used after in detail consultation with yourself
- This will be used as a last resort
- You will be made aware of the TGA mesh withdrawal in Australia and Europe – especially involving mesh used for vaginal prolapse surgery
- The sling is placed tension free.
- If you have a suspected Intrinsic Sphincter Deficiency (ISD), the sling may be placed tighter.
- The bladder will be inspected with a Cystoscopy to exclude any injuries to the bladder wall.
- The wounds are closed with dissolvable sutures and/or skin glue.
- A local anesthetic is given for pain relief.
- A urinary catheter is placed for 24hrs.
- A vaginal plug will also be placed.
- The catheter and plug will be removed early the next morning.
- The patient’s urine output will be measured each time they urinate, and the residual will be measured. (Patients will be required to do this up to 3 times).
- If the residual amount of urine is more than 250-300 cc, the patient may have to self-catheterize, until the residual volume is acceptable.
- Prophylactic antibiotics will be given to prevent infection.


What to expect after the procedure?
- Any anesthetic has its risks, and the anesthetist will explain all such risks.
- Complications:
- hemorrhaging, requiring blood transfusion <1%.
- bladder perforation, requiring an open repair <1%.
- Patients will wake up with a catheter in the urethra and bladder. This will remain in the bladder for 24 hrs.
- Above pubic bone area discomfort/pain will persist for a few days, but this will subside or settle.
- If you cannot urinate after 2-3 attempts, the sling may be readjusted.
- You may be required to self catheterize for a week or two.
- If there is no improvement the sling may be cut, to allow spontaneous urination.
- NB! Each person is unique and for this reason symptoms may vary!
What next?
- Patients will have a trial of void without catheter the next day.
- Patients will be discharged as soon as they can completely empty the bladder.
- Patients may be required to self-catheterize for a week or two.
- Patients may initially suffer from urge incontinence and frequency, but this will improve within the next 6 weeks.
- Your flow will be slower.
- Allow 6 weeks for symptoms to stabilize.
- There may be some blood in the urine. This can be remedied by drinking plenty of fluids until it clears.
- On discharge a prescription may be issued for patients to collect.
- Patients are to schedule a follow-up appointment in 6 weeks.
- Please direct all queries to Dr Schoeman’s rooms.
- PLEASE CONTACT THE HOPSITAL DIRECT WITH ANY POST-OPERATIVE CONCERNS AND RETURN TO THE HOSPITAL IMMEDIATELY SHOULD THERE BE ANY SIGNS OF SEPSIS.
Remember to discuss mesh and its complications with Jo. This is used as a last resort, and you should be aware of the risks!
Download Information Sheet


Leave a Reply
Want to join the discussion?Feel free to contribute!